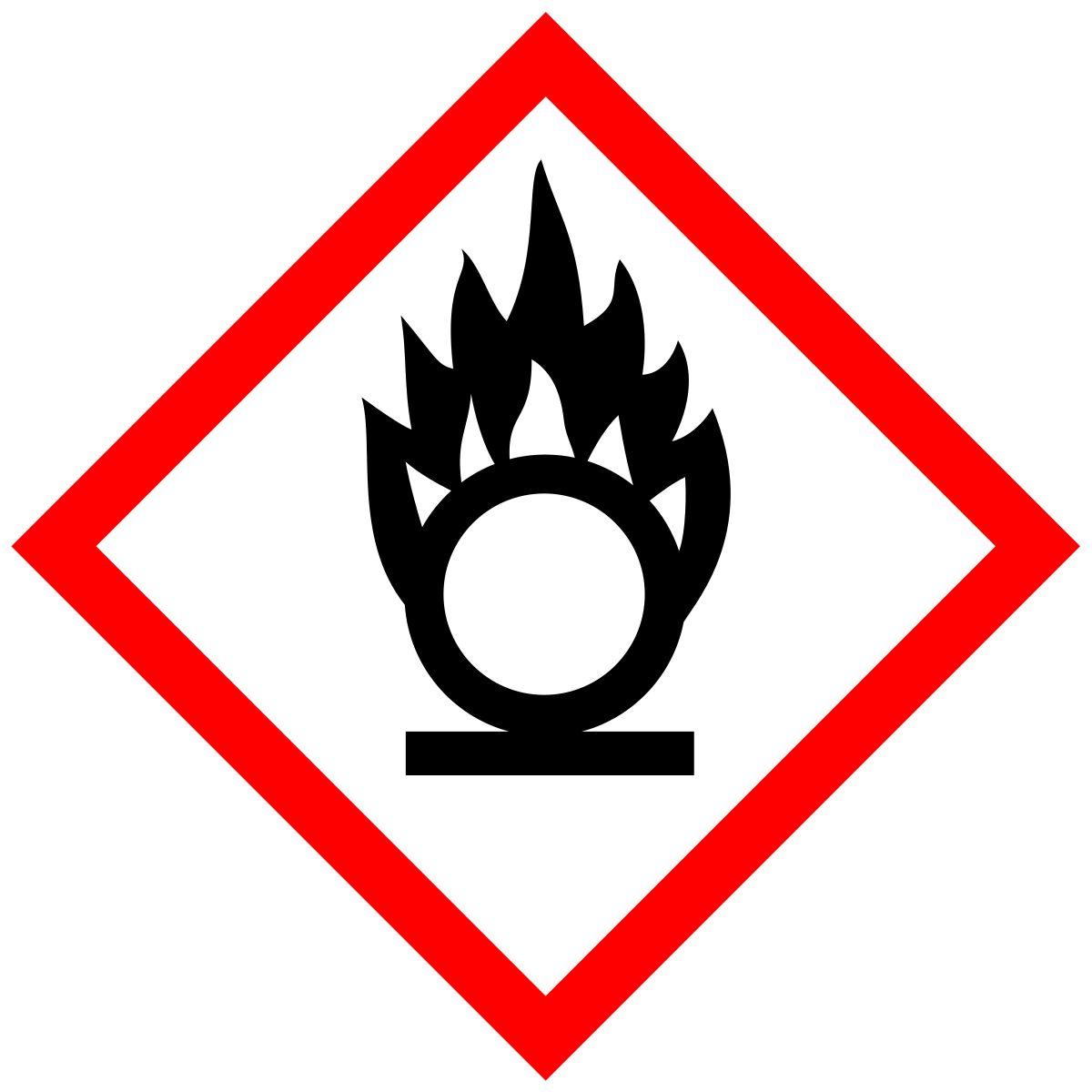
Oxidizing agent, 산화제
연료에 불이 붙게 하는 물질
극저온 액체 산소 사용하기도
Oxidizing agent
An oxidizing agent is a substance in a redox chemical reaction that gains or "accepts"/"receives" an electron from a reducing agent. In other words, an oxidizer is any substance that oxidizes another substance. The oxidation state, which describes the degree of loss of electrons, of the oxidizer decreases while that of the reductant increases; this is expressed by saying that oxidizers "undergo reduction" and "are reduced" while reducers "undergo oxidation" and "are oxidized".
Common oxidizing agents are oxygen, hydrogen peroxide, and the halogens.
https://en.wikipedia.org/wiki/Oxidizing_agent
Electron acceptor
An electron acceptor is a chemical entity that accepts electrons transferred to it from another compound.[1] It is an oxidizing agent that, by virtue of its accepting electrons, is itself reduced in the process. Electron acceptors are sometimes mistakenly called electron receptors.
https://en.wikipedia.org/wiki/Electron_acceptor
 Seonglae Cho
Seonglae Cho